About
- Activated Minerals® Technology
- Confidential Customer Support
- Discreet Packaging
- No Animal Testing or Animal Products
Doctor Recommended
Policies
FDA Compliance
Specials
Contact Us
Phone: 401-432-7750 (US) or 001-781-989-9586 (Int'l)
Email: support@aidance.com
Sitemap
Homeopathic Products & FDA
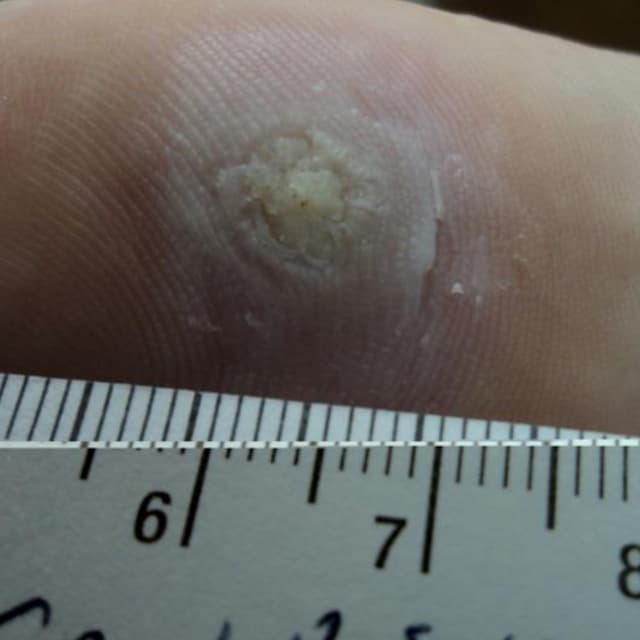
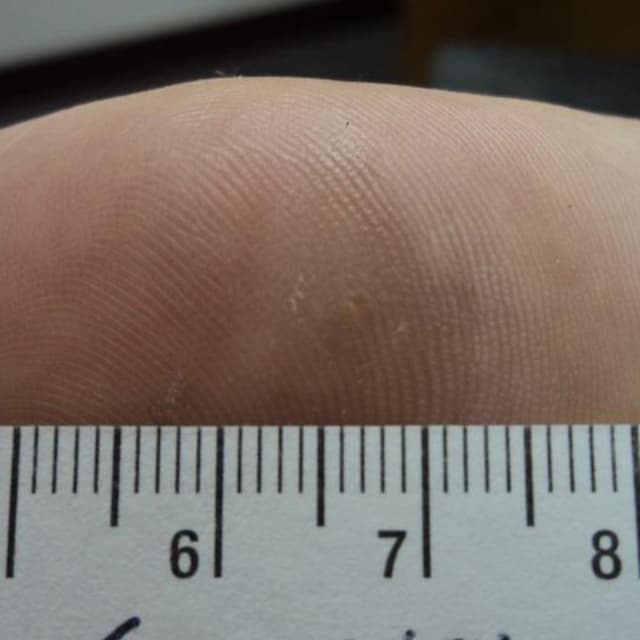
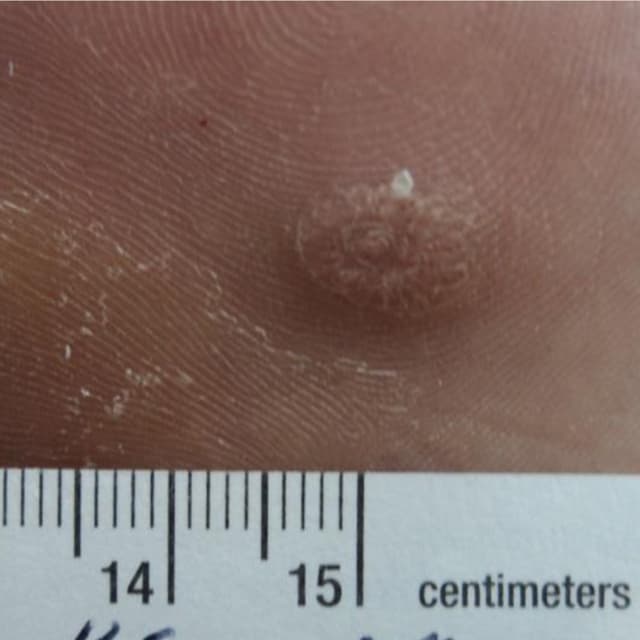
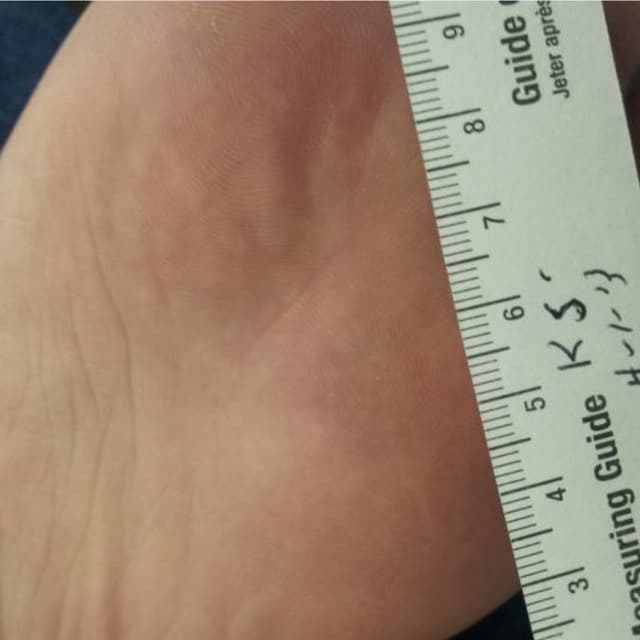
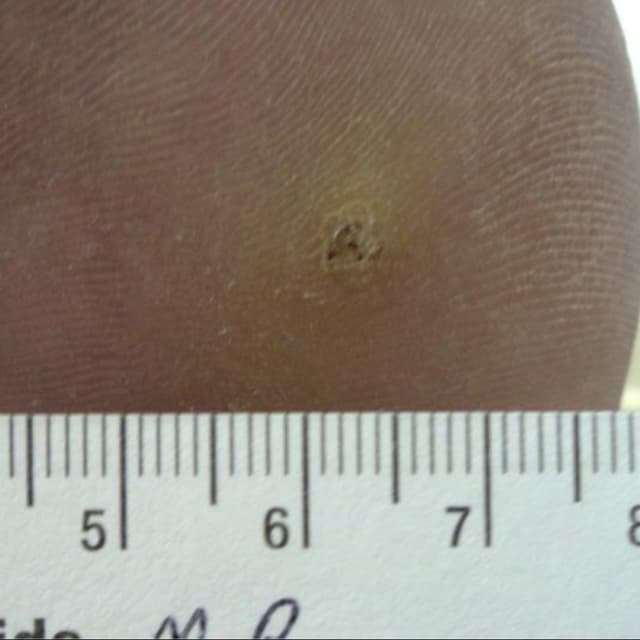
Terrasil Fast & Natural Wart Removal Ointment is a homeopathic formulation for wart symptom relief.
The FDA allows certain homeopathic formulations for sale which contain active ingredients that are listed in the Homeopathic Pharmacopoeia of the United States (HPUS), as our Terrasil wart care product does.
To be clear however, the FDA’s position on all homeopathic products is: “This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.”
The Drug Listing Act of 1972 requires registered drug establishments to provide the FDA with a current list of all drugs manufactured, prepared, propagated, compounded, or processed by it for commercial distribution.
These are known as National Drug Code, or NDC numbers. They are 10-digit, 3-segment numbers that are considered the universal product identifier.
Terrasil wart care ointment is an FDA-registered product, and as such, its NDC numbers are as follows: 24909-127-14, 24909-127-44
Terrasil wart removal ointment can be found in the FDA’s National Drug Code Directory here.